Electrometallurgical Process Is Used to Extract Which Metal
So option D is correct answer. In metallurgy this process is known as.
How Many Method Used In Extraction Of Metal Quora
Depending on the ions present in the acidic solutions different extractants are employed such as anionic cationic or solvating-type extractants.
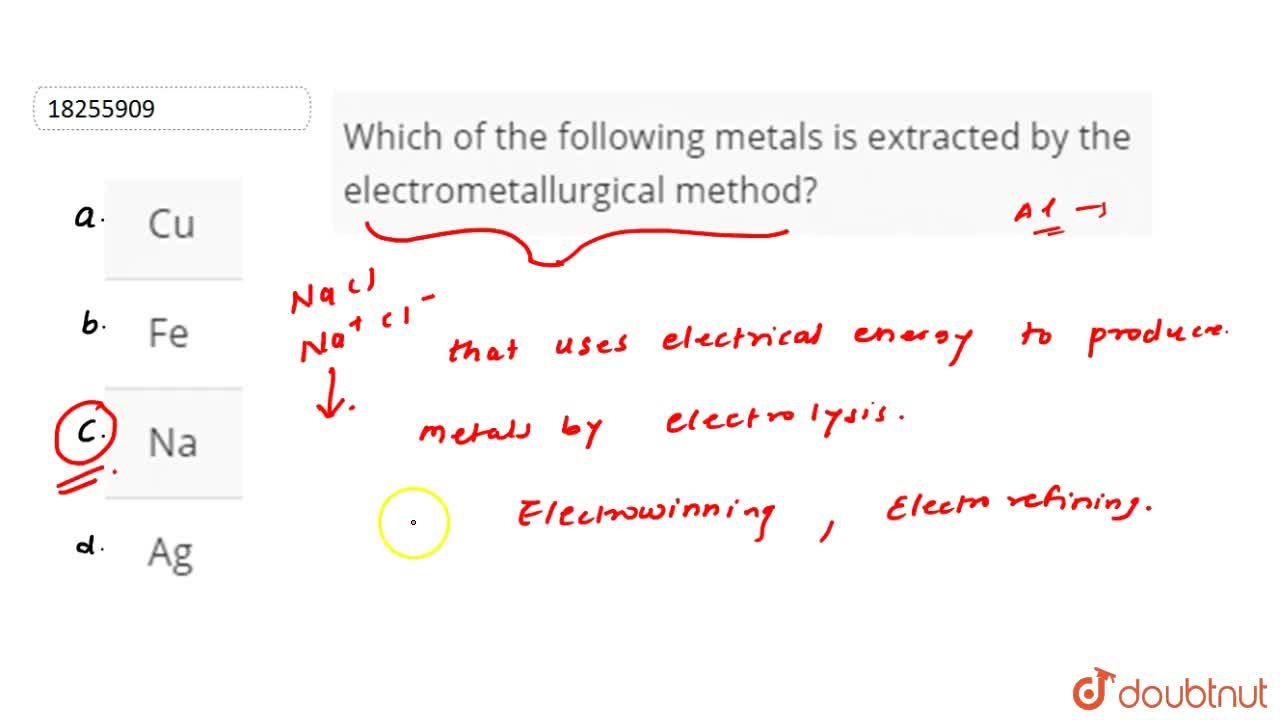
. Electrometallurgical method is used for the extraction of metals which are reactive in nature. Electrowinning is an electrochemical process employed to extract metals such as copper zinc nickel manganese and cadmium from their solutions. Which of the following metals are suitable for use as sacrificial anodes.
Main function of roasting is. Electro-refining is used to dissolve an impure metallic anode typically from a smelting process and produce a high purity cathode. They already exist in commercial practices Jha et al 2012.
So option B correct answer for this problem. Electrometallurgical process is used to extract. Electro-refining is used to dissolve an impure metallic anode typically from a smelting process and produce a high purity cathode.
The field is a materials science covering all aspects of the types of ore washing concentration separation chemical processes and extraction of pure metal and their alloying to suit various applications. For gold extraction the strength of cyanide solution ranges from 001 to 005. Electrometallurgical process is used to extract.
Pyrometallurgy which uses heat to start the separation process of metals from their mineral. Electrometallurgical process electrolysis of fused electrolyte is employed to extract. 15000000 Neodymium is an essential element for the powerful permanent magnets used wind turbines electric vehicles and other applications.
The part of metallurgy encompassing industrial methods for the production of metals and alloys by means of electric current. Electrometallurgy makes use of electrothermal and electrochemical processes. The anionic type is mostly amides or amines used for the extraction of vanadium gold iridium and sometimes rhodium and tungsten.
Innovative Process for Production of Neodymium Metal and Neodymium-Iron Master Alloy Amount. A iron b lead. Electrometallurgical methods of metal extraction is normally used for those metals_____.
AFe bPb cNa dAg. It is also called pyroprocessing and has. Electrometallurgy involves metallurgical processes that take place in some form of electrolytic cellThe most common types of electrometallurgical processes are electrowinning and electro-refiningElectrowinning is an electrolysis process used to recover metals in aqueous solution usually as the result of an ore having undergone one or more hydrometallurgical.
Electrothermal processes are used to extract metals from ores and concentrates and to produce and refine ferrous and nonferrous metals and alloys based. When lime stone is heated strongly it gives off CO2. Boston Electrometallurgical Corporation will develop and scale a one step molten oxide electrolysis process for producing Ti metal directly from the oxide.
Extractive metallurgy is a branch of metallurgical engineering wherein process and methods of extraction of metals from their natural mineral deposits are studied. ACalcination bRoasting cSmelting dOre dressing. Whose oxideore is not reduced by carbon.
Pyproprocessing is an electrometallurgical process in which spent fuel is dissolved in molten salt and uranium plutonium and other higher elements are partially separated though electrodeposition on a cathode. If the solution is rich enough can be treated by zinc powder and the precipitated obtained cement obtained will be sent to retorting and smelting. You would Electoral Light each employed to extract employed two extract sodium.
Electrolytic processing is used commercially to recover and refine metals including large-scale production of aluminum copper magnesium nickel and zinc as well as for recovery and refining of gold and silver. Electrometallurgical process is used to extract MNR 1985 89. Electrometallurgy this uses electric current to initiate the separation process of the metals.
Moreover by carefully choosing the leachants used it is possible to obtain a selective process maximizing the extraction of precious metals while minimizing the. Fused salt electrolysis is another electrometallurgical process whereby the valuable metal is dissolved into a molten salt which acts as the electrolyte and the valuable metal collects on the cathode of the cell. The electrolytic cell is the basic device used in this process consisting of an inert anode such as lead or titanium and a cathode placed in an aqueous electrolyte containing the metal solution.
ATo remove volatile substances bOxidation cReduction dSlag formation. Fused salt electrolysis is another electrometallurgical process whereby the valuable metal is dissolved into a molten salt which acts as the electrolyte and the valuable metal collects on the cathode of the cell. There are also exciting opportunities to utilize electrometallurgy in the production of titanium lead and other metals.
Sodiumcopperaluminium and many more. Electrometallurgical process is used to extract. Lime is added to the process in order to have a.
Titanium oxide is dissolved in a molten oxide where it is directly and efficiently extracted as molten titanium metal.
Why Is Electrolysis An Expensive Way To Extract Metal From Its Ore Quora

Which Of The Following Metals Is Extracted By The Electrometallurgical Method
No comments for "Electrometallurgical Process Is Used to Extract Which Metal"
Post a Comment